Copper Will Spontaneously Reduce Which of the Following
Cu2 2e- Cu E 034 V Ag e- Ag E 080 V If you could increase the concentration of Ag which of the following would be true about the cell potential. Copper will spontaneously reduce which of the following a Fe 2 and Ag b Fe 2 c from SCIENCE Chemistry at Bridgewater Raritan Regional High School.
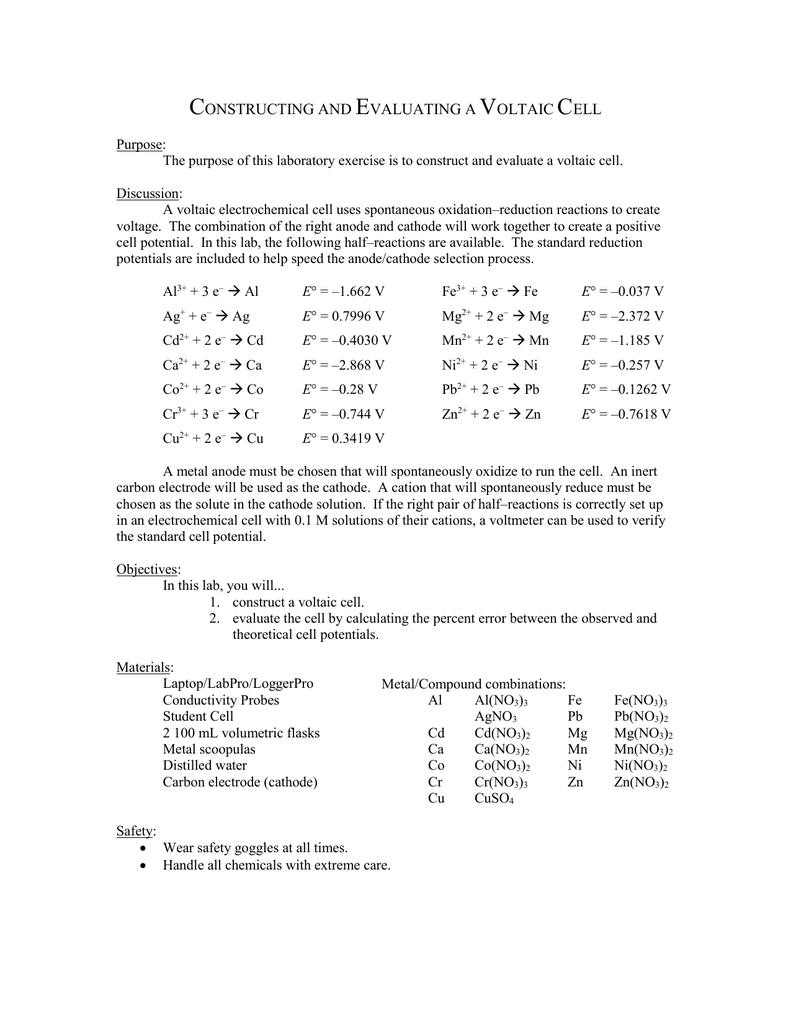
6 6 Constructing A Voltaic Cell Lab
Hence this is a spontaneous reaction.
. Base your answer on the following redox reaction which occurs spontaneously in an electrochemical cell. A strip of copper is placed in a 1 M solution of copper nitrate and a strip of silver is placed in a 1 M solution of silver nitrate. 136 gmL silver nitrate solution nitric acid solution.
The two metal strips are connected to a voltmeter by wires and a salt bridge connects the solutions. D None of these is true. For test tube 2 since the reduction potential of Sn is higher than Co Sn2 will reduce to Sns while Cos will oxidize to Co2.
Copper metal can be spontaneously oxidized by ceCu2 ions to form ceCu ions. To save 11 Chemistry - Science Dr. The atomic mass of copper is 63546.
Solid copper will spontaneously reduce which of the following a Fe 2 and Ag b Fe from CHEM 1465 at Keller High School. Write the balanced chemical equation for the reaction. Zinc copper mercury density.
Copper will spontaneously reduce which of the following A Fe 2 and Ag B Fe 2 C from CHEMISTRY 1211 at Georgia State University. This is a spontaneous reaction. Copper is electroplated from CuSO 4 solution.
Chemistry the value for the Ksp of silver chromate is reported to be 11 x 10-12. Table 201 Reaction ered_volts 287 F2 2e - 2F Clz Ag Cut 2e - 2C1 136 Ag 080 2e - Cu 034 Fe2 2e - -044 3e m AI 166 Natt Na -271 Fe2 and Ag 0 Fa7 end Al A3. How long will it take to deposit 100 10 2 g of Cu.
Agaq e- Ags ℰ 080 V. Please explain your reasoning and show your work. In a saturated solution of silver chromate the silver ion concentration is found to be 25 x 10-4M.
QUESTION 23 Copper will spontaneously reduce which of the following. B Al3 c Mg2 d Fe2 e Ag. The following standard reduction potentials apply.
Follow the steps provided in the simulation to add water to the graduated cylinder select one of the three samples copper silver or gold set its mass to the values given in the statements below find its volume and calculate its density. Using the data in Table 201 Copper will spontaneousl reduce which of the following. Given a 500-mL Erlenmeyer flask and a balloon can you combine two or more of the foregoing reagents to initiate a chemical.
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. CeCu ions will spontaneously form copper metal and ceCu2 ions in solution. B Fe2 C Fe2 and A Ag QUESTION 24 Choose the correct statement given the following information Fe3ag e- Fe2aq FeCD aa te FeCND aa O A Fo2tag is more lkely to be oxidized than Fe complexed to CN oB Fe3tag is more likely to be reduced than.
Silver oxide is used in small batteries called button batteries Figure below. You make a cell with a copper electrode in a solution of copper nitrate and a silver electrode in a solution of silver nitrate. Cu2 2e- Cu E 034 V Ag e- Ag E 080 V.
Consider the following reagents. For test tube 1 since the reduction potential of iron is higher than zinc iron will reduce to Fes and zinc will oxidize to Zn2. Why is the answer C and not B.
You make a cell with a copper electrode in a solution of copper nitrate and a silver electrode in a solution of silver nitrate. A The metal is a Lewis base and the ligands are Lewis acids. It would remain constant.
When copper wire is placed into a silver nitrate solution silver crystals and copperII nitrate solution form. C When the ligands approach a transition metal ion in an octahedral field the dxz dyz and dxy atomic orbitals are affected the least by the ligands. B Only complexes with coordination number six are found in nature.
Silver will spontaneously reduce which of the following. Zn Cr3 -- Zn2 Cr Write the half-reaction for the reduction that occurs. A Copper metal cannot spontaneously reduce any of these metal ions.
Copper metal will spontaneously reduce which of the following metal ions. Copper will spontaneously reduce which of the following a Fe 2 and Ag b Fe 2 c from CHM 101 at Grand Canyon University. None Which of the following will spontaneously oxidize N2H5 aq to N2g under standard conditions.
A constant current of 446 amp is applied by an external power supply. Type your answer using the format CH4 for CH4 1 Cus chemistry. Copper will spontaneously reduce which of the following a Fe 2 and Ag b Fe 2 c from CHEM 113 at Queens College CUNY.
Which of the following will spontaneously reduce N2g to N2H5aq under standard conditions. I thought that because copper is more likely to get oxidised than ceCu it is a better reducing agent and thus B is the answer. Of the metals Pb Mg Au and Na which will not spontaneously donate electrons to copper in solution.
What must the chromate ion concentration be.

Solved Nede Reaction E Volts Na E Na Al3 3e Al Chegg Com

Answered Based Off The Standard Reduction Bartleby

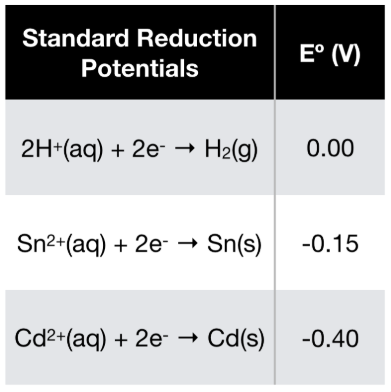
Consider The Following Cell Reaction At 298 K 2ag Cd 2ag Cd 2 The Standard Reduction Potentials E O For Ag Ag And Cd 2 Cd Are 0 80 V And
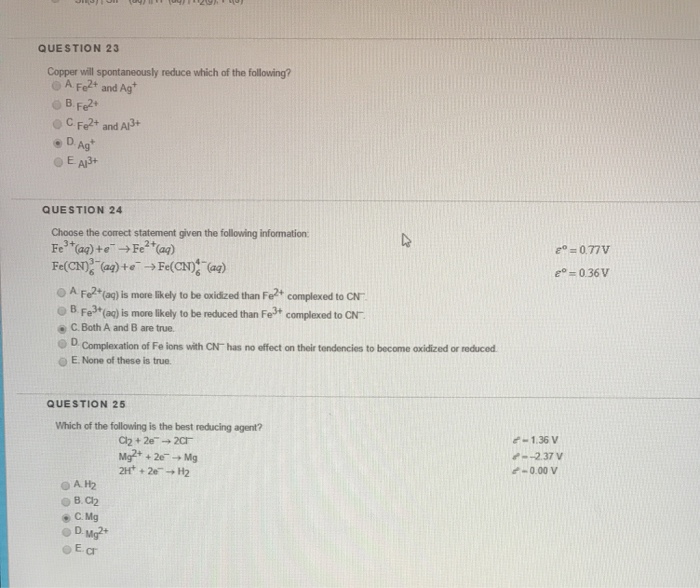
Solved Question 23 Copper Will Spontaneously Reduce Which Of Chegg Com

Answered Based Off The Standard Reduction Bartleby

Solved Use The Following To Answer Questions 4 5 G To Answer Chegg Com

Consider The Following Cell Reaction At 298 K 2ag Cd 2ag Cd 2 The Standard Reduction Potentials E O For Ag Ag And Cd 2 Cd Are 0 80 V And
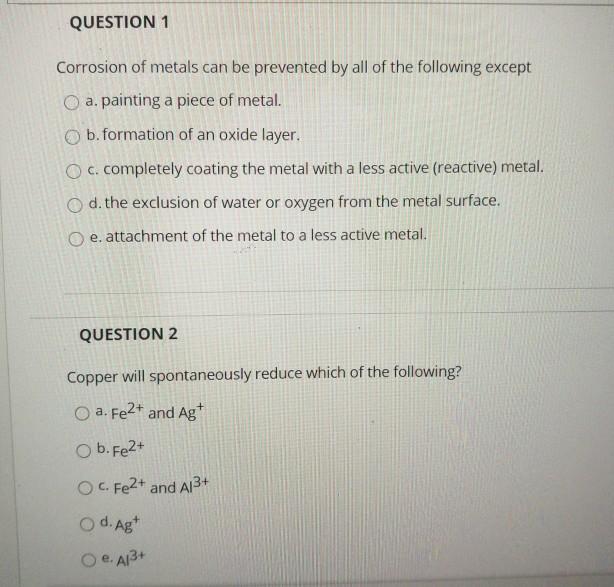
Solved Question 1 Corrosion Of Metals Can Be Prevented By Chegg Com

No comments for "Copper Will Spontaneously Reduce Which of the Following"
Post a Comment